FUNDAMENTALS
 Acoustic, or auditory, stimulation (AS) might contribute to alleviate motor and non-motor symptoms of Parkisnon Dissease and other neurodegenerative diseases, and therefore has the potential to be used as a co-adjuvant in the treatment of such diseases.
Acoustic, or auditory, stimulation (AS) might contribute to alleviate motor and non-motor symptoms of Parkisnon Dissease and other neurodegenerative diseases, and therefore has the potential to be used as a co-adjuvant in the treatment of such diseases.
Auditory stimulation at frequencies >30 Hz is proven to trigger an effect called entrainment, which is the coupling between the external stimulus and the internal oscillators of neural activity. The frequency of the stimulus is “mirrored” at the auditory cortex, and these oscillations, in turn, elicit others at different brain locations.
For example, auditory stimuli relate to motor functions through basal ganglia-thalamocortical and cerebellar-thalamocortical networks, as well as to cognitive functions through the mesolimbic system (e.g. memory, emotions and reward-related motor function learning) and hypothalamic-pituitary-adrenal axis (e.g. cardiovascular and endocrine responses). The fact that the brain connectivity during entrainment occurs via thalamus suggests that auditory stimuli may compensate the lack of endogenous oscillations at the basal ganglia due to neurodegeneration.
This paradigm is supported by theoretical and mathematical models of neural activity and plasticity between the auditory and motor brain networks. Several studies have been published where different auditory stimulation protocols have been tested in healthy subjects and in Alzheimer’s and Parkinson’s patients.
OUR WORK: CLINICAL AND PRE-CLINICAL STUDIES
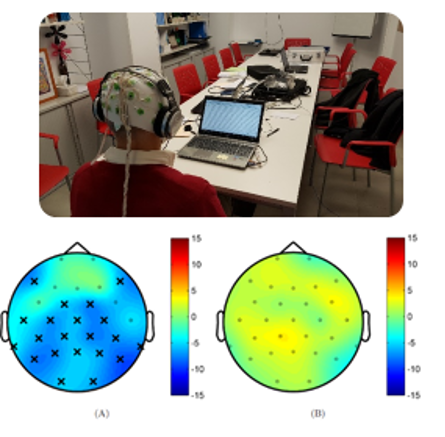 In the Neuroacoustics Lab we have conducted both pre-clinical and clinical studies about the effects of auditory stimulation with healthy subjects and patients with several neurological disorders, although we have worked more intensively in Parkinson disease. As shown in the figure, we have observed that different auditory stimulations produce different changes in the rain activity of patients with PD. In both cases the figure shows the difference in brain activity in the theta band before and after an stimulation session. In the left part we observe the effect of an stimulus that was designed to reduce the power in this band, whereas in the right we observe the effect of a neutral stimulus. This preliminary results are supported by theoretical and mathematical models of neural activity and plasticity between the auditory and motor brain networks. And other groups have been published similar studies where different auditory stimulation protocols have been tested in healthy subjects and in Alzheimer’s and Parkinson’s patients. But there are still a lot of open questions, such as: Which stimulation frequencies and protocols are the most appropriate for each case, and what are their effects? Is there an habituation effect or could we use this stimulation in a long term therapeutic intervention? Could it be used in any ND and stage of the disease, or only in certain groups of patients? These are some of the questions we are trying to answer.
In the Neuroacoustics Lab we have conducted both pre-clinical and clinical studies about the effects of auditory stimulation with healthy subjects and patients with several neurological disorders, although we have worked more intensively in Parkinson disease. As shown in the figure, we have observed that different auditory stimulations produce different changes in the rain activity of patients with PD. In both cases the figure shows the difference in brain activity in the theta band before and after an stimulation session. In the left part we observe the effect of an stimulus that was designed to reduce the power in this band, whereas in the right we observe the effect of a neutral stimulus. This preliminary results are supported by theoretical and mathematical models of neural activity and plasticity between the auditory and motor brain networks. And other groups have been published similar studies where different auditory stimulation protocols have been tested in healthy subjects and in Alzheimer’s and Parkinson’s patients. But there are still a lot of open questions, such as: Which stimulation frequencies and protocols are the most appropriate for each case, and what are their effects? Is there an habituation effect or could we use this stimulation in a long term therapeutic intervention? Could it be used in any ND and stage of the disease, or only in certain groups of patients? These are some of the questions we are trying to answer.
In order to do so we are also working with healthy and animal model of Parkinson’s disease, as this might help us to understand better the underlying mechanisms behind the observed effects of auditoy stimulation, and also to optimize the stimulation protocols. We are using behavioural tests, electrophysiology recordings (EEG and EMG) and histology to characterize the possible functional alterations present in the brain and to study motor and non-motor effects of each type of stimulation protocol.
A LAB WITH A TRANSLATIONAL AIM
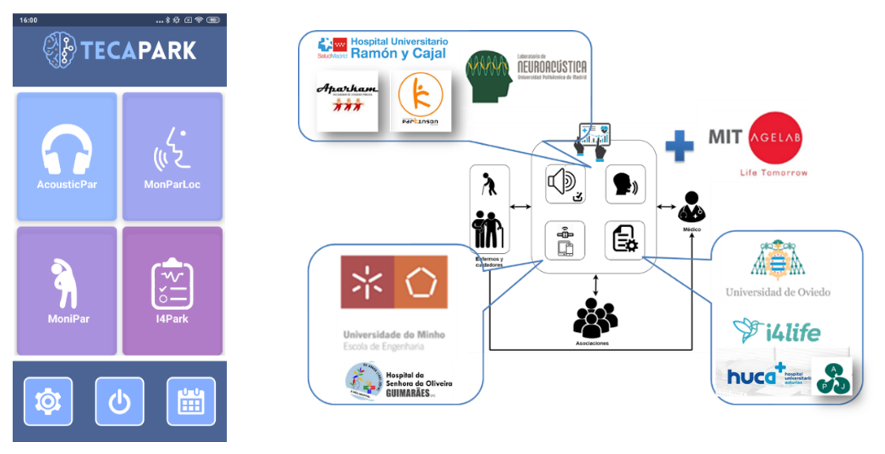
The goal of our lab is to combine this fundamental research with our other research activities and capabilities to produce meaningful, applicable results that can improve the life of those affected by theses diseases. This means that we also work in specific projects where we analyze the possible benefits of applying these technologies in real work scenarios. A good example of such, has been the Teca-Park projectwhere we developed and tested an App to perform studies of acoustic stimulation remotely using wearables and smart phones to track the evolution of motor and non-motor symptoms of PD patients.
The App had four software modules: one for acoustic stimulation, and three to monitor motor and non-motor symptom’s using data from a smartwatch (acelerometry), a smartphones (voice) and standardized questionnaires. This system was used by 30 patients with PD during 3 months with the collaboration of 5 patient assotiations and under the supervision of three neurologists from Spain and Portugal.
You can read more about this project in http://www.i2a2.upm.es/tecapark/